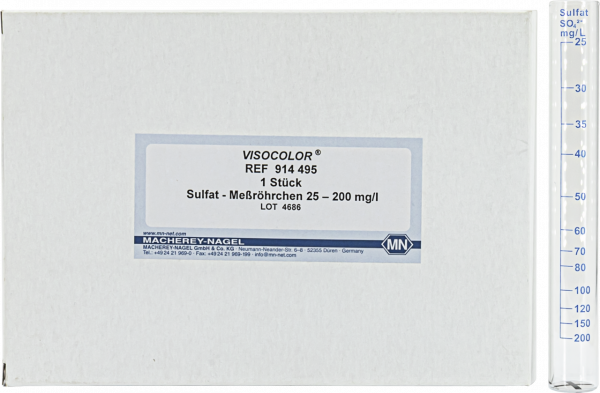
Measuring tube for VISOCOLOR ECO Sulfate
*taxes and shipping not included
Delivery time approx. 5 working days
Spare measuring tube for VISOCOLOR ECO Sulfate
| Brand | VISOCOLOR |
| Platform | Accessories |
| Scope of delivery | 1 measuring tube |
| Gross weight (incl. packaging) | 41.74 g / 0.09 lbs |
| Packaging dimensions | 122 x 90 x 45 mm / 4.80 x 3.54 x 1.77 Inch |
| Hazardous material | No |

Sulfate
Alongside sulfide (S2-) and sulfite (SO32-), sulfate (SO42-) is one of the most prominent sulfur compounds. sulfate is formed by deprotonation of sulfuric acid or is present as an anion of salts. As a component of numerous natural minerals, such as gypsum or alum, sulfate is omnipresent in nature.
How to perform the NANOCOLOR Sulfate HR 1000 tube test
General information
Sulfate is also frequently found naturally in bodies of water, where it is formed by the dissolution of rocks and minerals. In Germany, a limit value of 250 mg/L SO42- applies to drinking water, as higher levels promote corrosion and lead to a bitter taste.
sulfuric acid and sulfates are used in a variety of industrial applications, for example in fertilizer production, where they serve as important nutrients for plants. Sulfates also play a central role in the chemical industry, as they are used as starting materials for the production of various chemicals. In addition, sulfates are used in water treatment to remove impurities and improve water quality. They are also important in the construction industry, as they are used in cement and other building materials.
A high sulfate concentration can therefore indicate possible pollution of the water caused by industrial wastewater or agricultural practices. sulfates in high concentrations are harmful to the environment. Thus, monitoring sulfate concentrations helps in water management and ecosystem protection to assess the impact of human activities on natural water resources. At wastewater treatment plants, sulfates in wastewater can lead to chemical reactions that can affect the efficiency of the plant and promote the formation of harmful by-products
Reaction basis
The sulfate concentration in NANOCOLOR and VISOCOLOR is determined by precipitation with barium(II) and subsequent turbidity measurement. Only freely dissolved sulfate is detected.
To ensure a precise and reliable determination, it is important that the barium sulfate is finely distributed in the cuvette in the correct particle size. Precise adherence to the instructions prevents these errors. In particular, the reaction time must be strictly adhered to in order to obtain reliable results.
| Ba2+ + SO42- → BaSO4↓ |
Product portfolio
The high-precision NANOCOLOR range covers a wide concentration range with NANOCOLOR Sulfate LR 200, NANOCOLOR Sulfate MR 400, NANOCOLOR Sulfate HR 1000, all in accordance with the APHA 4500-SO42- E standard. NANOCOLOR Sulfate HR 1000 contains a formulation patented according to EP3346269B1.
We offer various standards for internal quality control: NANOCONTROL Multistandard Drinking Water, NANOCONTROL Multistandard Metals 1 and especially NANOCONTROL Sulfate LR 200.
VISOCOLOR ECO Sulfate can be evaluated both photometrically and visually. The refill pack is sufficient for photometric determination.

Determination is particularly quick and easy with VISOCOLOR Powder Pillows Sulfate. The addition of just one solid reagent simplifies the procedure and avoids errors.


Preservation of samples
- The samples should be stored in a cool place. It is not necessary to adjust the pH value. The sample can be stored for one day.
Tips and tricks
Digestion
- Only freely dissolved sulfate is determined. Digestion is not provided.
Background information
- If there is uncertainty about the concentration in the sample to be analyzed, a preliminary test with QUANTOFIX Sulfate (REF 91329) provides quick information. The required dilution for the determination can be recognized from this and applied directly.
Seawater analysis
- Also suitable for the determination of sulphate in seawater with appropriate dilution in the measuring range.
pH value
- The pH values of the sample solution specified in the package inserts must be observed. If necessary, the pH value should be adjusted with nitric acid or caustic soda.
Interferences
- Turbidity interferes particularly sensitively with turbidity detection.
- High calcium and carbonate contents can also lead to turbidity formation.
- Further interfering ions are listed in the package inserts.
Turbidity
- Turbid samples lead to incorrect measurement results and must be filtered before performing the test. The formation of turbidity during the addition of reagents is desirable, however, as this is a turbidity test.
Filtration
- For coarsely dispersed turbidity, filter with high-quality filter paper (e.g. MN 615), for medium-dispersed turbidity with glass fiber paper (e.g. MN 85/70 BF) or membrane filtration set GF/PET 0.45 µm, for finely dispersed turbidity with membrane filtration set 0.45 µm or GF/PET 0.45 µm.