Standard test NANOCOLOR Nitrite
*taxes and shipping not included
Delivery time approx. 5 working days
Standard test for the determination of Nitrite. Reagent kit for highly sensitive photometric measurements with low costs per sample. Pre-programmed on NANOCOLOR photometers.
| Brand | NANOCOLOR |
| Platform | NANOCOLOR standard tests |
| Parameter | Nitrite |
| Measuring range | Nitrite - 0.002–0.30 mg/L NO₂-N, Nitrite - 0.005–1.00 mg/L NO₂⁻ |
| Sludge reagent set | No |
| Test No. | 1-67 |
| Evaluable on | 500 D, Advance, MT Easy UV, MT Easy VIS, UV/VIS II, VIS II |
| Based on norm | Analogous to APHA 4500-NO2- B, DIN EN 26777 DIN EN 26777 – D10 |
| Method | Nitrite - Sulfanilic acid/1 -naphthylamine |
| NanOx N | No |
| NanOx Metal | No |
| Crack-Set | No |
| Sea water analysis | Yes |
| Application | wastewater, water |
| Test range | Low |
| Test method | Photometric |
| Remark | Measuring range on NANOCOLOR VIS II |
| Shelf life (from production) | 1.5 Year(s) |
| Storage temperature | 15–25 °C / 59–77 °F |
| Scope of delivery | Rugged box with reagents for 250 tests. |
| Gross weight (incl. packaging) | 550.23 g / 1.21 lbs |
| Packaging dimensions | 160 x 110 x 130 mm / 6.30 x 4.33 x 5.12 Inch |
| Hazardous material | Yes |
- Download Instruction / Anleitung / Manuel
- Download Info Simplified working procedure (EN)
- Download Info Vereinfachte Durchführung (DE)
- Download Validation data / Validierungsdaten / Données de validation
- Download Betriebsanweisung (DE)
- Download Flyer NANOCOLOR analysis system (EN)
- Download Flyer Das NANOCOLOR Analysensystem (DE)
- Download Flyer Système d’analyse NANOCOLOR (FR)

Nitrite
Nitrite ions are present in low concentrations in surface waters and rarely in groundwaters. In wastewaters, by contrast, nitrite concentrations are often higher. Nitrite ions are present in water almost exclusively in dissolved form. Higher nitrite concentrations in wastewaters suggest either industrial effluents (metal pickling agents, chemical industry) or fecal contamination (decomposition product of nitrification: nitrite is produced during the bacterial oxidation of ammonia and in the reduction of nitrate).
How to perform the NANOCOLOR Nitrite tube test
General information
Another source of nitrite formation from nitrate are of galvanized iron pipes of domestic installations. Concentrations up to 1 mg/L are still classified as harmless. A major field of application of nitrites is the manufacture of dyes.
In adults, reduction of nitrate to nitrite takes place in the small intestine, whereas in infants this occurs already in the stomach. Nitrites are toxic to humans. They block the blood pigment hemoglobin, which is necessary for oxygen transport. Especially in
infants, life-threatening conditions with acute suffocation may occur (infant cyanosis = methemoglobinaemia). In addition, nitrites are also involved in the formation of carcinogenic nitrosamines.
Nitrites are toxic since they block the blood pigment hemoglobin and thus the transport of oxygen.
Nitrites have high and pH-dependent toxicity to fish.
According to the German Drinking Water Ordinance (TrinkwV), limits are 0.1 mg/L in the outflow of sewage treatment plants, and 0.5 mg/L in the tap.
Despite their toxicity, nitrites have numerous applications. They are used e.g. as food additives or in the production of sausages as nitrite curing salt. In general, an increase in nitrite content is always a sign of bacterial contamination. These can be detected early by a nitrite measurement, which is also an important parameter in cooling lubricants.
Nitrites react with cooling lubricants to form carcinogenic nitrosamines, which can enter the human body as aerosols.
NO₂-N is frequently used as notation rather than NO₂⁻, which indicates that only the mass of nitrogen of the nitrite ion is taken for account while the oxygen atoms are neglected. The conversion factor of NO₂-N to NO₂⁻ is 3.28, resulting from the atomic
masses.
| mg/L NO₂⁻ | mg/L NO₂-N |
|---|---|
| 0.02 | 0.006 |
| 0.05 | 0.015 |
| 0.1 | 0.03 |
| 0.2 | 0.06 |
| 0.5 | 0.15 |
Reaction basis
Depending on the product range (VISOCOLOR or NANOCOLOR) and test kit, one of two different reactions is underlying:
(a) ISO method in analogy to ISO 6777:
Nitrite reacts with sulfanilamide and N-(1-naphthyl)-ethylenediamine to form a purple azo dye. The underlying reaction is analogous to DIN EN 26777-D10, APHA 4500-NH2 and EPA 354.1.
Sample preservation
By adjusting the pH to 1-2 with sulfuric acid, the sample can be stored for up to 7 days (storage vessel: PE bottle). Ideally, storage and transport are carried out at 4 °C in the dark.
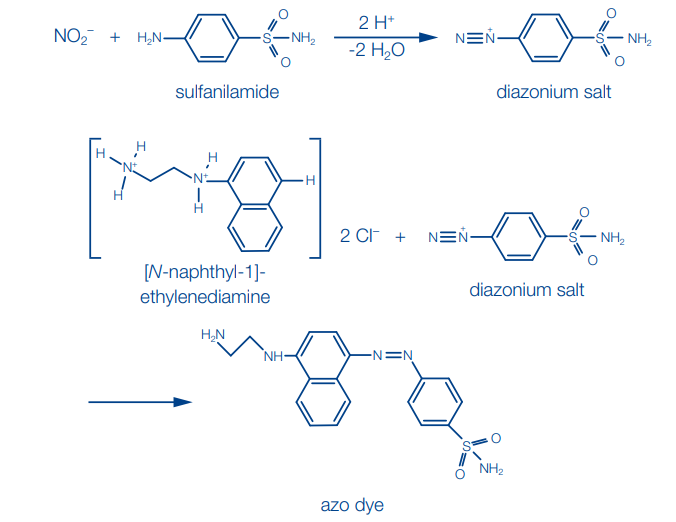
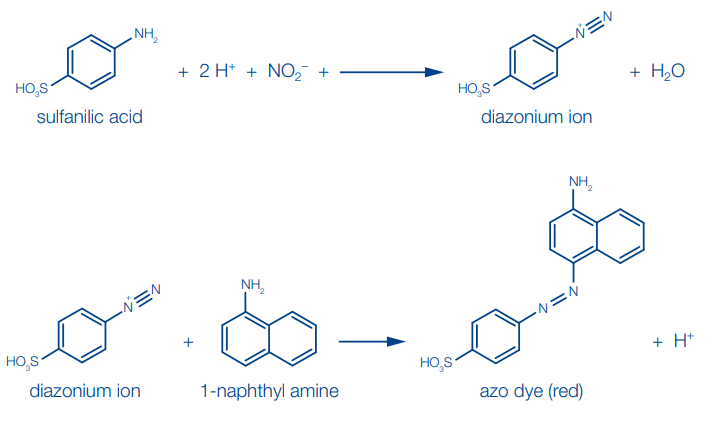
(b) Sulfanilic acid method (Griess-Ilosvay reaction):
Sulfanilic acid is diazotized by nitrite in acidic solution. The diazonium salt which is formed is then coupled with 1-naphthylamine to form a red azo dye.
Tips & tricks
Background information
The reagents for sample preparation by clarification precipitation (REF 918937) can be used for removal of emulsions, turbidity and color prior to the nitrite determination, e.g. in cooling lubricants, landfill leachate, etc.
Additionally a blank value should be measured for nitrite measurements in heavily contaminated samples (e.g. in inlet waters).
Sea water suitability
All VISOCOLOR and NANOCOLOR nitrite tests are suitable for seawater analysis.
pH
The pH values for the sample solution stated in the instruction leaflet must be complied with. If necessary, adjust the pH with hydrochloric acid or sodium hydroxide.
Interferences
Organic colloids, chlorine, humic acids and colored heavy metal ions interfere. Further interfering ions are listed in the instruction leaflets.
Turbidity
Turbid samples have to be filtered; turbidity leads to incorrect results: For coarsely dispersed turbidities, use qualitative filter paper (e.g. MN 615), for moderately dispersed turbidities, use glass-fiber paper (e.g. MN 85/70 BF) or membrane filtration set GF/PET 0.45 µm, for finely dispersed turbidities, use membrane filtration kit 0.45 µm or GF/PET 0.45 µm.