Tube test NANOCOLOR COD 160
*taxes and shipping not included
Delivery time approx. 5 working days
Tube test for the determination of COD. Precise rapid tests for all kind of water and waste water samples according to ISO 15705. Time-saving and reliable analysis together with our NANOCOLOR photometers.
| Brand | NANOCOLOR |
| Platform | NANOCOLOR tube tests |
| Parameter | COD |
| Measuring range | COD - 15–160 mg/L O₂ |
| Sludge reagent set | No |
| Test No. | 0-26 |
| Evaluable on | 500 D, Advance, MT Easy UV, MT Easy VIS, PF-3 COD, PF-12Plus, UV/VIS II, VIS II |
| Based on norm | In accordance with DIN ISO 15705 – H45, analogous to EPA 410.4, APHA 5220 D, DIN 38409 – H41-1 |
| Method | COD - Potassium dichromate |
| NanOx N | No |
| NanOx Metal | No |
| Crack-Set | No |
| Sea water analysis | No |
| Remark | Measuring range on NANOCOLOR VIS II |
| Shelf life (from production) | 1 Year(s) |
| Storage temperature | 15–25 °C / 59–77 °F |
| Scope of delivery | Rugged box with 20 test tubes. Sufficient for 20 tests. |
| Gross weight (incl. packaging) | 376 g / 0.83 lbs |
| Packaging dimensions | 160 x 111 x 122 mm / 6.30 x 4.37 x 4.80 Inch |
| Hazardous material | Yes |
- Download Instruction / Anleitung / Manuel
- Download Pictogram / Piktogramm / Pictogramme
- Download Validation data / Validierungsdaten / Données de validation
- Download Betriebsanweisung (DE)
- Download Flyer NANOCOLOR analysis system (EN)
- Download Flyer Das NANOCOLOR Analysensystem (DE)
- Download Flyer Système d’analyse NANOCOLOR (FR)
COD - Chemical oxygen demand
COD is an effect parameter
COD is not a quantity-based size but an effect parameter (a requirement), as is illustrated in the following example:
| K2Cr2O7 + 8 H+ → 3 <O> + 2 Cr3+ + 2 K+ + 4 H2O |
In acid medium, potassium dichromate forms reactive oxygen species, by the reaction with hydrogen ions.
| CH3OH + 3 <O> → CO2 + 2 H2O |
These oxygen compounds are able to oxidize organic compounds such as ethanol or methanol to carbon dioxide.
| CH4 + 4 <O> → CO2 + 2 H2O |
The consumption of oxidizing agent is independent of the size of the molecule. For example, the oxidation of methanol consumes less oxidant than that of the smaller methane, since less oxygen is consumed in the reaction to carbon dioxide and water. This results in a larger theoretical COD for methane than for methanol. The COD content can be determined from the consumption of potassium dichromate. The evaluation is done photometrically or by titration. Depending on the measurement range, the decrease in concentration of the yellow potassium dichromate or the increase in concentration of the green chromium(III) ion is determined. For all tests with a low measurement range, up to the test NANOCOLOR COD 300 (REF 985033), the former applies. From the test NANOCOLOR COD 600 (REF 985030) on, the increase of the green chromium(III) is determined. An important process in COD determination is decomposition. In analogy to DIN 38409- H41, decomposition is carried out for two hours at 148 °C. A faster decomposition at 160 °C for 30 minutes is also possible. However, this rapid decomposition is not suitable for all tests. Please refer to the instruction leaflet of the respective test. COD decomposition in a microwave oven, in analogy to decompositions with NANOCOLOR NanOx N and NANOCOLOR NanOx Metal, is not permitted for safety reasons.
The COD covers all oxidizable contents of the sample.
K2Cr2O7 = potassium dichromate
H+ = hydrogen ion
Cr3+ = Cr(III) ion
K+ = potassium ion
<O> = oxygen compounds
H2O = water
Reaction basis
The chemical oxygen demand of a water is determined by silver-catalyzed oxidation (increase in the oxidizability of aliphatic substances) with potassium dichromate / sulfuric acid for 2 hours at 148 °C. The COD covers all oxidizable contents of the sample. In COD testing with a low measurement range, the decrease of the potassium dichromate concentration is determined; in COD tests with a higher measurement range, by contrast, the increase in Cr(III) ion concentration is determined. Possibly present chloride is precipitated by mercury sulfate. Thus it is removed from the unwanted oxidation to elemental chlorine. Silver sulfate serves as a catalyst to increase the oxidizability of aliphatic substances. Thus lower apparent findings are avoided. The underlying reaction is analogous to APHA 5220-D, EPA 410.4 and DIN 38409-H41. Furthermore, seven NANOCOLOR COD tests meet the requirements of the DIN ISO 15705:2002 standard. The main reaction is described in the following equation with potassium hydrogen phthalate (KHP), which serves as a reference substance:
| 2 KC8H5O4 + 10 K2Cr2O7 + 41 H2SO4 → 16 CO2 + 46 H2O + 10 Cr2(SO4)3 + 11 K2SO4 |
Since each molecule of potassium dichromate K2Cr2O7 has the same oxidizing power as 1.5 O2 molecules, the equivalent reaction is:
| 2 KC8H5O4 + 15 O2 + H2SO4 → 16 CO2 + 6 H2O + K2SO4 |
As described above, two molecules of KHP consume 15 oxygen molecules. Therefore, the theoretical COD for one milligram KHP is 1.175 milligrams of oxygen O2.
Advantages of the NANOCOLOR analysis system compared to DIN 38 409 H41
- Reduced amounts of toxic mercury
- Generally lower quantities of toxic and hazardous reagents
- All reagents are pre-filled in the test tubes
- Significantly reduced risk of accidents for the user
- Reproducible results thanks to photometric evaluation
Tips & tricks
Sample preservation
The sample should be thoroughly mixed or homogenized in a blender to be as representative as possible for the COD of the water to be examined.
COD determinations should be performed as soon as possible after sampling. If this is not possible, the pH should be reduced to 2 or less by the addition of 2 mL of concentrated sulfuric acid H2SO4 per 1 L of sample.
Storage of the acidified sample at room temperature: Analysis within 7 days.
Storage of the acidified sample at 4 °C: Analysis within 28 days.
Decomposition
Rapid decomposition is not possible for all COD tests. Depending on the composition of the test, the water content can be higher. This results in a higher vapor pressure during the decomposition and increases the risk for bursting of the test tube. For more information about decomposition, please see the respective instruction leaflet.
Background information
PHP (potassium hydrogen phthalate) is used as a reference substance.
NANOCOLOR COD tube tests have advantages over the German norm DIN 38409-H41 in terms of use of toxic chemical compounds and user-friendly handling. Furthermore, seven NANOCOLOR COD tests meet the requirements of the DIN ISO 15705:2002 standard.
Filtration
For cumulative parameters, the sample must not be filtered prior to decomposition! By filtration, compounds which are poorly soluble are removed. Those compounds would thereby escape the detection which would lead to wrong results.
For a determination of dissolved COD, filtration of turbid samples using the membrane filtration kit 0.45 µm (REF 91650 / 91652) or glass fiber paper MN 85/90 BF is recommended. These filters are tested and validated specifically for this purpose.
Sea water suitability
The analysis of seawater is possible with the test COD 60 in salt water (REF 985020).
The test allows COD determinations at chloride concentrations from 2 to 18 g/L.

Fig. 1: Concentration series of our COD NANOCOLOR tube test with increasing COD concentration.
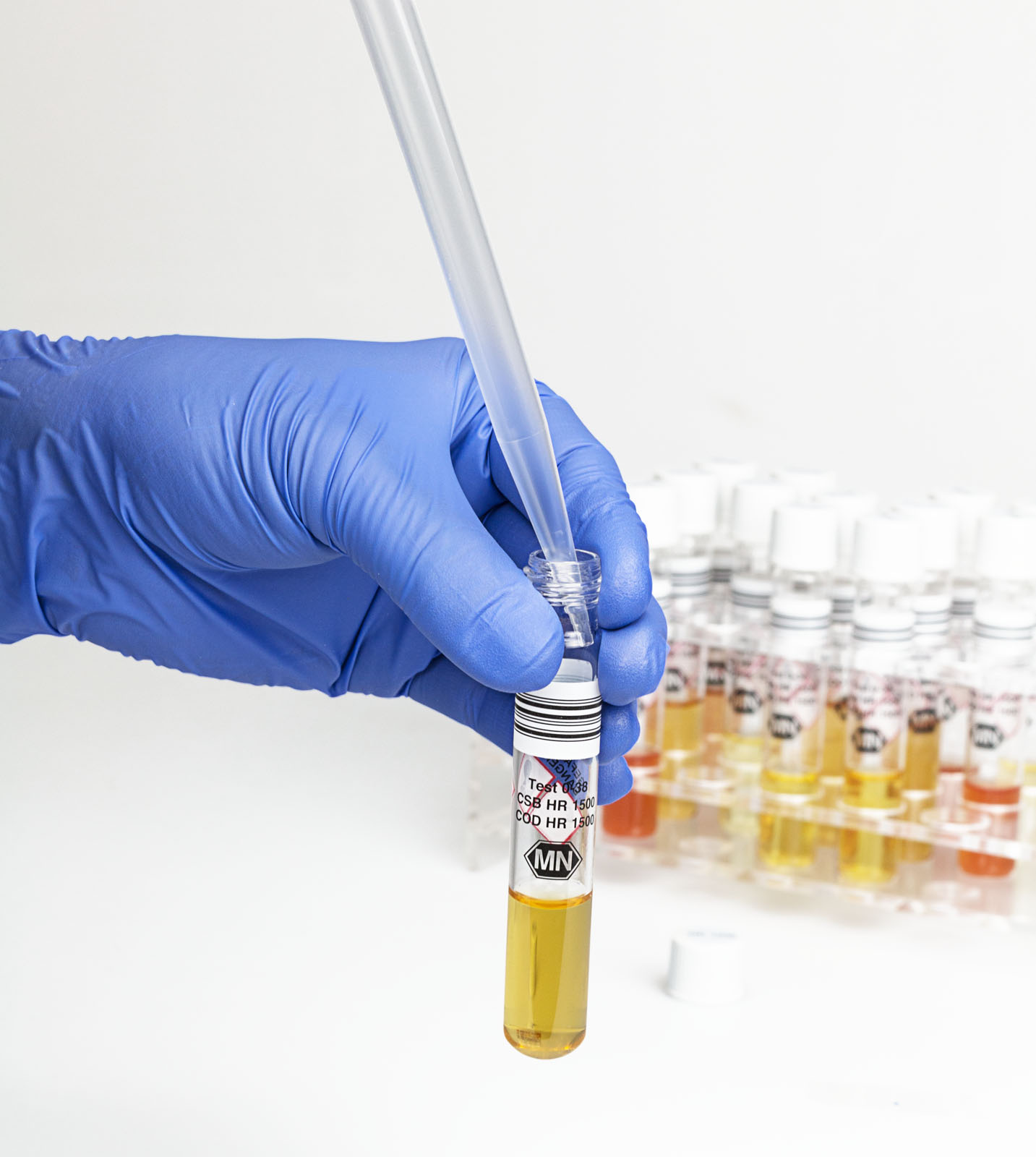
Fig. 2: Depicted procedure of the COD determination and tube-handling with our easy-to-use and safe NANOCOLOR tube tests.
How to perform the NANOCOLOR COD tube test
The chemical oxygen demand (COD) is one of the most important parameters for the assessment of industrial and municipal wastewater. As a cumulative parameter, COD determines all chemically oxidizable components present in water. Hence, this includes not only biodegradable substances (as in the BOD5), but also chemical compounds that cannot be determined by biological oxidation (e.g. nitrogen compounds such as nitrites). It has also the advantage of a faster availability compared to BOD. The COD indicates the contamination of a water sample. It is therefore also used as an evaluation parameter to determine pollution units in discharges under the German Wastewater Charges Act. By definition (ISO 15705), the COD is the concentration of oxygen that is equivalent to the mass of potassium dichromate that is consumed in total oxidation of organic substances present in the water sample. Mercury sulfate and silver sulfate as well as sulfuric acid are listed as auxiliary reagents.
Watch the webinar recording on how to become an expert in COD analysis here: