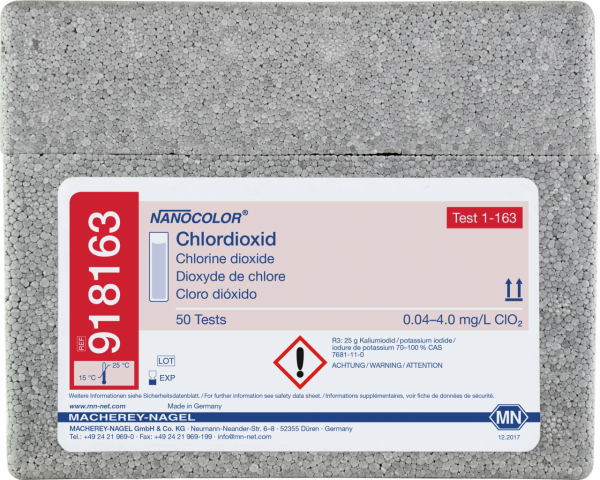
Standard test NANOCOLOR Chlorine dioxide
*taxes and shipping not included
Delivery time approx. 5 working days
Standard test for the determination of Chlorine dioxide. Reagent kit for highly sensitive photometric measurements with low costs per sample. Pre-programmed on NANOCOLOR photometers.
| Brand | NANOCOLOR |
| Platform | NANOCOLOR standard tests |
| Parameter | Chlorine dioxide |
| Measuring range | Chlorine dioxide - 0.04–4.00 mg/L |
| Sludge reagent set | No |
| Test No. | 1-163 |
| Evaluable on | 500 D, Advance, UV/VIS II, VIS II |
| Based on norm | Analogous to APHA 4500-ClO2 D, DIN 38408 G5 |
| Method | Chlordioxide - DPD |
| NanOx N | No |
| NanOx Metal | No |
| Crack-Set | No |
| Sea water analysis | Yes |
| Remark | Measuring range on NANOCOLOR VIS II |
| Shelf life (from production) | 1.5 Year(s) |
| Storage temperature | 15–25 °C / 59–77 °F |
| Scope of delivery | Rugged box with reagents for 50 tests. |
| Gross weight (incl. packaging) | 471 g / 1.04 lbs |
| Packaging dimensions | 160 x 110 x 130 mm / 6.30 x 4.33 x 5.12 Inch |
| Hazardous material | Yes |
Chlorine - a highly reactive element
Chlorine is a chemical element in the category of halogens. It is naturally occurring as the gas Cl2 and is one of the most reactive elements on earth. It is highly oxidative. Chlorine and its derivatives are the most important disinfectants for disinfection of swimming pools, water mains, and water reservoirs. Electroplaters use chlorine for the detoxification of cyanide-containing waste. Regular monitoring of chlorine level is essential as excessive chlorine not only impairs the smell and taste of water but also can be hazardous.
One distinguishes between free chlorine and combined chlorine (chloroamines); the sum of both is called total chlorine.
What is chlorine?
Free Chlorine
Free chlorine is the sum of two highly reactive and disinfecting substances, which form when chlorine gas dissolves in water.
| Cl2 + H20 → HClO + H+ + Cl- HClO → H+ + OCl- |
The disinfection process with free chlorine in water is called chlorination. It is commonly used in water with low organic pollutants. It is applied for the prevention of algal growth, for control of taste and odor in food, removal of elements like iron and manganese, decomposition of hydrogen sulfides or cyanides and improvements of coagulation. In terms of disinfection, chlorination is used in samples with low concentrations of hard-to-treat microorganisms, e.g. giardia or cryptosporidium.
Combined Chlorine (Chloramines)
Chloramines, commonly known as combined chlorine, form when free chlorine is added to water containing ammonia. Chloramines are still usable for disinfection purposes, therefore this reaction can be wanted in water treatment. There are three species of chloramines depending on how much chlorine is reaction with the ammonia-ion. These are monochloramine, dichloramine and nitrogen trichloride.
| HClO + NH3 → NH2Cl + H2O HClO + NH2Cl → NHCl2 + H2O HClO + NHCl2→ NCl3 + H2O |
The use of chloramines in disinfection is called chloramination. Because chloramines have less oxidation potential and a lower reactivity than free chlorine, they also form less side products with organic matter during disinfection. This can be beneficial, as free chlorine can form disinfection by-products such trihalomethanes. Those disinfection by-products are called DBPs and can be carcinogenic. Therefore, there is a general trend to replace chlorination with free chlorine by chloramination. In addition, the demand of chlorine is less in chloramination, due to less side reactions, which saves cost.
Total Chlorine
The sum of all free and combined chlorine species is called total chlorine. It is often determined to evaluate the level of combined chlorine by subtraction, because there is not direct measurement of combined chlorine available.
HOCl = Hypochlorous acid
OCl- = Hypochlorite ion
NH2Cl = monochloramine
NHCl2 = dichloramine
NCl3 = trichloramine
Total chlorine = free chlorine + combined chlorine
How to measure Chlorine
Chlorine is generally monitored by colorimetric methods. By the addition of certain reagents and buffers, chlorine reacts to form a colored product. The intensity of this color is proportional to the chlorine concentration. The color intensity is then determined visually, reflectometrically or photometrically with compact or spectro photometers.
Test strips - Dip & Read
The easiest and fastest way to perform chlorine measurements are Dip & Read tests. These test strips combine fast performance with accurate results. They are based on a chemical reaction between chlorine and a dye. A redox reaction takes place and the test pads develops a certain color intensity depending on the concentration of chlorine in the sample.
Different test strips are used to perform evaluation of free or total chlorine.
MACHEREY-NAGEL offers different test strips and test papers mainly in the QUANTOFIX and DiaQuant series.
Colorimetric test kits - Easy DPD tests
DPD (N,N-Diethyl-p-phenylendiamine) is the most commonly used substance in colorimetric chlorine test kits. It reacts with free chlorine to yield an intensively pink colored dye. The intensity of the dye is proportional to the free chlorine concentration.
In visual, colorimitric tests, this pink-colored sample is then compared to a color scale to determine the right concentration.

Fig. 1: Concentration series of our NANOCOLOR Chlorine/ozone 2 tube test with increasing chlorine concentration.
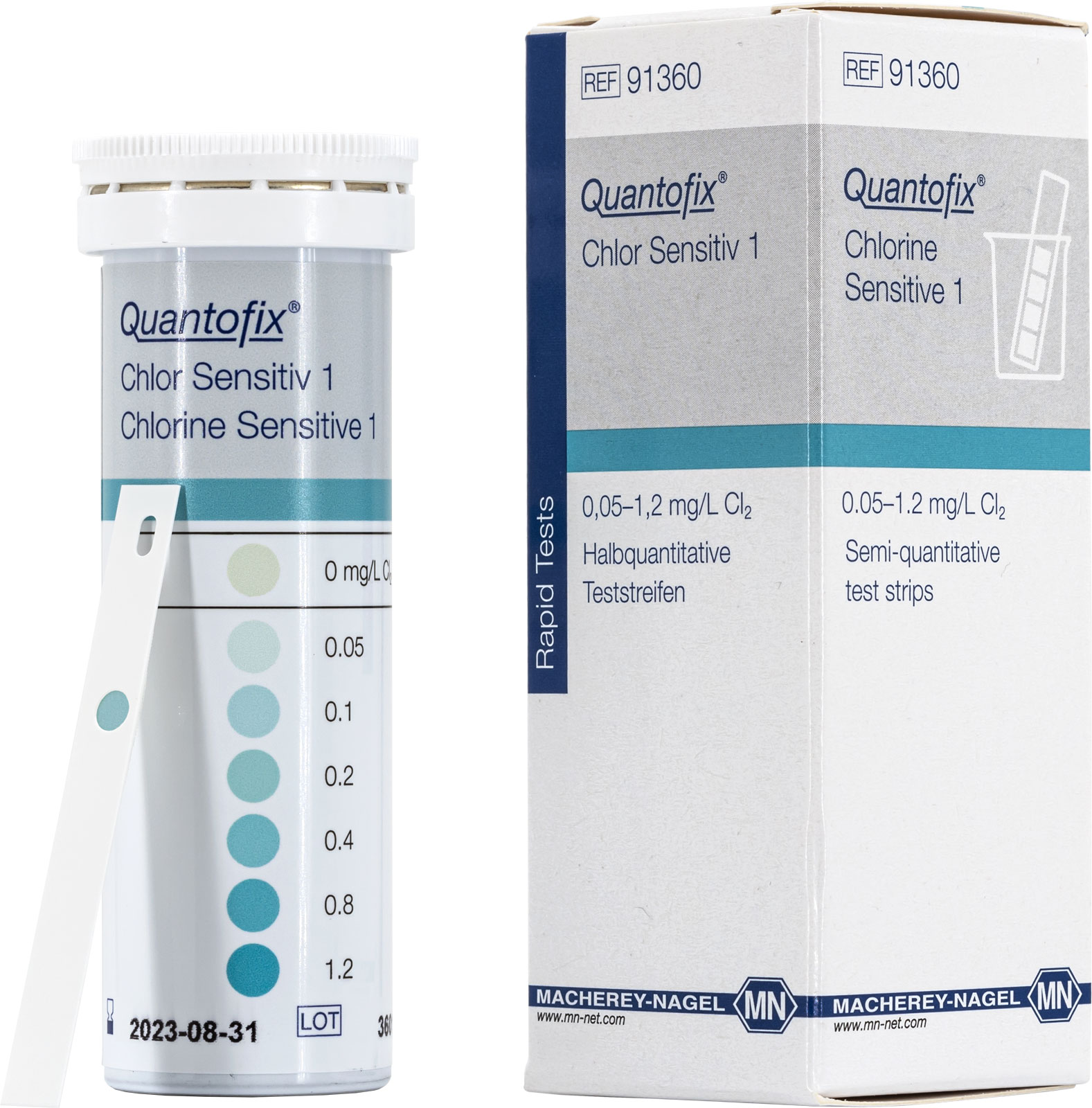
Chlorine analysis with QUANTOFIX Chlorine sensitive 1 test strips
Photometric test kits - Precision analysis
Photometric test kits use the same DPD chemistry like colorimtetric test kits. But instead of a visual determination by eye, a photometer is used. It can detect the color intensity of the solution more accurate than the user. This enhances precision and accuracy in chlorine analysis.
MACHEREY-NAGEL offers photometric chlorine test kits in the NANOCOLOR and VISOCOLOR product ranges.
How to measure chlorine content with VISOCOLOR Powder Pillows Chlorine free
Where is Chlorine monitored?
Drinking water treatment plants and distribution
Chlorine is one of the most frequently used disinfectants in drinking water treatment. It also helps in precipitation of minerals and other matter by oxidation before filtration. In many countries and regions worldwide drinking water has to be disinfected before distribution. This is done after the filtration by a repeated addition of chlorine. The chlorine levels have to be monitored, together with pH-value and temperature, at many stages of the process.
Depending on microorganisms present in the water, a residual chlorine level is adjusted before distributing the drinking water to customers. International norms carefully regulate the tolerable level of chlorine in drinking water. National laws might also restrict the maximum level of chlorine present in drinking water at the tap. National drinking water acts vary greatly in maximum chlorine concentrations allowed. Whereas German Trinkwasserverordnung mandates 0.3 mg/L, US-EPA sets 4.0 mg/L as the upper limit.
Besides disinfection, chlorine also serves for the elimination of sulfides, which causes a rotten-egg smell, ammonia and nitrogen-compounds, which can cause an unpleasant smell. Chlorine also removes iron and manganese which can cause a bitter taste and coloration of the drinking water.
In contrast to the many positive effects of chlorination, chlorine can also reacts with organic substances in the water to form so-called disinfection-by-products (DBP) which can be harmful to human health. This is another reason why chlorine levels have to be monitored carefully during drinking water production.
Waster water treatment plants
In many countries, chlorination is used as the final stage in waste water treatment. In this case the treated water is disinfected with chlorine before discharge into the receiving waters. The reason for the chlorination is the prevention of waterborne diseases from pathogens and microorganisms from the previous treatment.
A thorough dechlorination process is very important to reduce harmful effects to the receiving waters by residual chlorine in the effluent.


Industrial cooling systems
Industrial cooling systems, like cooling towers, are susceptive to algae growth and the formation of bio-films within the tubing. This bio-groth can clog the water systems. Chlorine is used to effectively prevent the bio-growth. But it has to be monitored closely, as too-low concentrations do not prevent bio-growth and too-high concentrations of chlorine can cause corrosion and damage.