Tube test NANOCOLOR ortho- and total-Phosphate LR 1

*taxes and shipping not included
Delivery time approx. 5 working days
Tube test for the determination of ortho- and total Phosphate. Precise rapid tests for all kind of water and waste water samples. Time-saving and reliable analysis together with our NANOCOLOR photometers.
| Brand | NANOCOLOR |
| Platform | NANOCOLOR tube tests |
| Parameter | Phosphate (ortho), Phosphate (total) |
| Measuring range | Phosphate (ortho) - 0.05–0.50 mg/L P , Phosphate (ortho) - 0.2–1.5 mg/L PO₄³⁻ , Phosphate (total) - 0.05–0.50 mg/L P , Phosphate (total) - 0.2–1.5 mg/L PO₄³⁻ |
| Sludge reagent set | No |
| Test No. | 0-95 |
| Evaluable on | 500 D, Advance, MT Easy UV, MT Easy VIS, PF-12Plus, UV/VIS II, VIS II |
| Based on norm | Analogous to EPA 365.2+3, APHA 4500-P E, DIN EN ISO 6878 – D11 |
| Method | Phosphate - Phosphomolybdenum blue |
| NanOx N | No |
| NanOx Metal | Yes |
| Crack-Set | No |
| Sea water analysis | Yes |
| Remark | Measuring range on NANOCOLOR VIS II |
| Shelf life (from production) | 1 Year(s) |
| Storage temperature | 15–25 °C / 59–77 °F |
| Scope of delivery | Rugged box with 20 test tubes. Sufficient for 20 tests. |
| Gross weight (incl. packaging) | 378.3 g / 0.83 lbs |
| Packaging dimensions | 160 x 110 x 119 mm / 6.30 x 4.33 x 4.69 Inch |
| Hazardous material | Yes |
- Download Instruction / Anleitung / Manuel
- Download Pictogram / Piktogramm / Pictogramme
- Download Info Bestimmung kondensierter Phoshpate (DE)
- Download Validation data / Validierungsdaten / Données de validation
- Download Betriebsanweisung (DE)
- Download Flyer NANOCOLOR analysis system (EN)
- Download Flyer Das NANOCOLOR Analysensystem (DE)
- Download Flyer Système d’analyse NANOCOLOR (FR)

Phosphate (PO43-)
Inorganic and organic phosphorus compounds are now found in almost all municipal and industrial wastewaters. They are predominantly present in the form of ortho-, polyor organo-phosphates. The commonality is the basic structure of phosphate, PO43-.
How to perform the NANOCOLOR Phosphate tube test
Together with nitrogen and potassium compounds, phosphates are among the most important fertilizers for adequate plant growth. In addition, phosphates are added as detergent additives for water softening and as preservatives in foodstuffs, since they inhibit growth of fungi and bacteria. However, their use as a water softener is declining, since too high phosphate levels in waters lead to over-fertilization and ultimately to “collapse”.
H3PO4 = ortho-phosphoric acid
H4P2O7 = diphosphoric acid
Moreover, natural phosphate resources are limited. Therefore, increasingly efforts are made to recover phosphorus from sewage sludge in wastewater treatment plants. This is done primarily with biological phosphorus removal, since the availability of phosphorus in its natural form is not unlimited.
Phosphates are important fertilizers.
The phosphate content of surface water determines its trophic status. Very high phosphate levels lead to eutrophication (over-fertilization: increased growth of algae and aquatic plants) of rivers and lakes and can ultimately lead to extinction of plants and fish. Pure waters, e.g. mountain streams, have phosphate levels of < 0.1 mg/L PO43-, usually even less than 0.03 mg/L PO43-. Concentrations above 0.1 mg/L PO43- are only connected to pollution only if other pollution parameters are positive as well. Phosphate levels above 0.3 mg/LPO43- are an indication of pollution (special cases are swamp waters with levels of up to 1 mg/L PO43-). A high phosphate content is a reliable chemical indicator in case of fecal contaminations. Even normal levels may be determined in polluted deeper groundwaters, because some soils can absorb phosphates.
Phosphates are of importance for pipe networks with aggressive waters, due to the formation of protective coatings. However, for adequate formation 0.1 mg/L (max. 1 mg/L) are already sufficient.
A general distinction has to be made between inorganic phosphorus compounds (phosphateor hydrogen phosphate ions and polyphosphates) and organically bound phosphorus. Polyphosphates and organically bound phosphorus can be determined only after oxidative decomposition (“destruction”).
Phosphates play an important role for humans in bone formation in the form of calcium phosphate. They are also important in energy metabolism. However as little phosphate as possible should be present in drinking water, since excessive amounts can lead to digestive upset and are suspected of triggering kidney problems as well.
The main entry of phosphates is with municipal wastewaters (cleaning products) and agriculture (fertilizers). Phosphates must be removed from wastewater, since higher amounts will result in a eutrophication of the receiving water.
The phosphate content of surface water determines its trophic status.
Phosphates can be eliminated chemically or biologically:
In chemical phosphorus precipitation, soluble phosphates are converted into insoluble compounds by the addition of suitable precipitating agents and are thus removed from the solution by precipitation. Iron chlorides and sulfates, aluminum salts or lime are used as precipitation agents.
Phosphates can be eliminated chemically or biologically.
In biological phosphorus removal (Bio-P), micro-organisms are used. They are deprived of oxygen in an anaerobic basin. They release stored phosphates for energy production to survive. When the micro-organisms are then supplied with oxygen again, they absorb the previously released phosphates and other phosphates in the solution. Hence, the phosphate content in the basin is reduced.
Further notations are used in addition to the phosphate ion (PO43-) in many tests. The notation PO4-P is often used which reflects only the phosphorus content and disregards the oxygen content, comparable to the nitrogen parameters ammonium, nitrate and nitrite. The conversion factor of PO4–P to PO43- is 3.07.
In condensation reactions under heating, ortho-phosphate reacts to form its polymeric acids and salts:
| 2 H3PO4 → Δ T → H4P2O7 + H2O |
General information
The differences between the individual units for one parameter result from the differences in molar masses.
This difference is illustrated using the example of phosphate. The phosphate ion PO43- consists of one phosphorus atom and four oxygen atoms. If the unit PO43- is used, the concentration of the complete phosphate ion (including the oxygen atoms) is determined.
If, however, PO4-P is used as unit, only the P portion is used for evaluation, i.e. without the four oxygen atoms. Without the four oxygen atoms, the ion is much lighter, and thus the measurement value is lower. The following figure illustrates this once more:
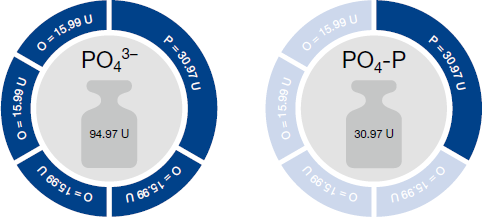
U = g/mol
Determination of the conversion factors:
| PO4-P / PO43- = 30.97 U / 94.97 U = 0.3 |
For example:
| 1.5 mg/L PO43- x 0.33 = 0.5 mg/L PO4-P |
| 0.5 mg/L PO4-P x 3-07 = 1.5 mg/L PO43- |
You can choose the desired unit in the photometers directly after selecting the test by choosing the corresponding sub-method. Always pay attention to the unit shown in the display in order to avoid possible errors from an incorrect unit. In the photometer manuals and pictograms, all programmed sub-methods are listed for the individual tests.
Units
| mg/L PO43- | mg/L P2O5 | mg/L PO4-P |
|---|---|---|
| 0.6 | 0.5 | 0.2 |
| 1.5 | 1.1 | 0.5 |
| 3 | 2 | 1 |
| 6 | 5 | 2 |
| 15 | 12 | 5 |
Conversion table mg/L PO43-, mg/L P2O5 and mg/L PO4-P
Further notations are used in addition to the phosphate ion (PO43-) in many tests. The notation PO4-P is often used which reflects only the phosphorus content and disregards the oxygen content, comparable to the nitrogen parameters ammonium, nitrate and nitrite. The conversion factor of PO4–P to PO43- is 3.07.
(a) DIN method in analogy to DIN EN ISO 6878-D11, APHA 4500-PE as well as EPA 365.2 and 365.3.: Ammonium molybdate reacts with ortho-phosphate ions to form phosphomolybdic acid.
| PO43- + 12 MoO42- + 24 H+ + 3 NH4+ → (NH4)3[P(Mo3O10)4] + 12 H2O |
This is reduced with a reducing agent to phosphorus molybdenum
blue. Acidic oxidation at 100–120 °C is performed for the determination of
total phosphate, to include poly- and organophosphates.
| (NH4)3[P(Mo3O10)4] + Na2S2O5 → phosphorus molybdenum blue |
PO43- = phosphate ion
MoO42- = molybdenum(VI) oxide
NH4+ = ammonium ion
(NH4)3[P(Mo3O10)4] = phosphomolybdic acid
Na2S2O5 = sodium disulfite
Mo4O10(OH)2 = molybdenum blue
Reaction basis
Depending on the product range (VISOCOLOR or NANOCOLOR) and test kit, one of two different reactions is underlying:
(b) Vanadate method: ortho-phosphate ions react with molybdate and vanadate ions to form a yellow phosphoric acid/molybdate/vanadate complex. The underlying reaction is also analogous to APHA 4500-P C.
| PO43- + 2 VO3- + 10 MoO42- + 10 H2O → [PV2Mo10O40]5- + 20 OH- |

VO3- = vanadate ion
[PV2Mo10O40]5- = phosphoric acid / molybdate / vanadate complex
- The sample can be stored for up to 7 days by adjusting the pH to 1-2 with sulfuric acid. (Storage vessel: PE bottle). Ideally, storage and transport are carried out at 4 °C in the dark.
- Generally, cooling of the sample is recommended.
- The phosphate portion, which is not present as ortho-phosphate, occurs in sewage treatment plants mostly in the form of sludge particles. The total phosphate content is thus affected by the content of suspended matter. Accordingly, a sample representative with respect to the suspended matter must be taken. In addition, a careful homogenization of the sample (by Ultra-Turrax® or magnetic stirrer) must be performed prior to determination. Any cooled samples should be brought to room temperature before homogenization. Homogenized samples must be analyzed immediately!
- If only the ortho-phosphate content of a sample containing suspended matter is to be determined, it must be filtered as quickly as possible.
Sample preservation
Decomposition
- Decomposition with NANOCOLOR NanOx Metal is recommended in case of an elevated content of organic materials and/or organically bound phosphorus.
- An incomplete decomposition leads to lower apparent results and possibly to lower total phosphate than ortho-phosphate levels.
Filtration
- For cumulative parameters, the sample must not be filtered prior to decomposition! Compounds which are poorly soluble are removed by filtration. Those compounds would thereby escape a detection which would lead to wrong results.
- In the determination of ortho-phosphate, on the other hand, filtration is performed prior to analysis. Turbid samples have to be filtered; turbidity leads to incorrect results: For coarsely dispersed turbidities, use qualitative filter paper (e.g. MN 615), for moderately dispersed turbidities, use glass-fiber paper (e.g. MN 85/70 BF) or membrane filtration set GF/PET 0.45 μm, for finely dispersed turbidities, use membrane filtration kit 0.45 μm or GF/PET 0.45 μm.
- Any precipitates which are present after the decomposition can be removed using membrane filters before determination.
For cumulative parameters, the sample must not be filtered prior to decomposition.
Background information
- Difference between ortho- and total phosphate:
- Ortho-phosphates are readily soluble, usually inorganic salts of phosphoric acid. These compounds are directly accessible to analysis and do not require a decomposition.
- In addition to the readily soluble ortho-phosphates also poly- and organo-phosphates are determined in total phosphate determination. The latter are not directly accessible to the analysis reaction. The poly- and organo-phosphates are converted into ortho-phosphates by an acidic decomposition at 100–120 °C and can thus be detected as well. These hard-to-oxidize phosphate compounds can be decomposed with NANOCOLOR® NanOx Metal.
- Generally, for determination of phosphate with our products, the phosphates must be present as ortho-phosphates.
Ortho-phosphates are directly amenable to analysis. For the determination of total phosphate, previous decomposition is required.
Homogenization
- The use of an inhomogeneous sample for a determination of total phosphate can lead to underestimated results.
Sea water suitability
- All VISOCOLOR and NANOCOLOR phosphate tests are suitable for seawater analysis.
pH
- The pH values of the sample solution that are stated in the package inserts must be complied with. If necessary, adjust the pH with sulfuric acid.
Interferences
- Any interference by silica can be eliminated by the addition of citric acid.
- Further interfering ions are listed in the instruction leaflet.
Turbidity
- Turbid samples have to be filtered; turbidity leads to incorrect results: For coarsely dispersed turbidities, use qualitative filter paper (e.g. MN 615), for moderately dispersed turbidities, use glass-fiber paper (e.g. MN 85/70 BF) or membrane filtration set GF/PET 0.45 μm, for finely dispersed turbidities, use membrane filtration kit 0.45 μm or GF/PET 0.45 μm.
- Turbidity can lead to apparently lower total phosphate than ortho-phosphate content.
Common sources of errors
-
Carryover of phosphoric acid (e.g. from the tube test NANOCOLOR Nitrate 50): Nitrate is measured frequently before the determination of phosphate. The nitrate test comprises phosphoric acid. The failure not to change the pipette tip may result in carryover and thus in higher apparent phosphate. A carryover takes place even if only the top edge of the nitrate test tube has touched the pipette tip.













