Tube test NANOCOLOR Nitrate 8
*taxes and shipping not included
Delivery time approx. 5 working days
Tube test for the determination of Nitrate. Precise rapid tests for all kind of water and waste water samples. Time-saving and reliable analysis together with our NANOCOLOR photometers.
| Brand | NANOCOLOR |
| Platform | NANOCOLOR tube tests |
| Parameter | Nitrate |
| Measuring range | Nitrate - 0.30–8.00 mg/L NO₃-N, Nitrate - 1.3–35.0 mg/L NO₃⁻ |
| Sludge reagent set | No |
| Test No. | 0-65 |
| Evaluable on | 500 D, Advance, MT Easy UV, MT Easy VIS, PF-12Plus, UV/VIS II, VIS II |
| Based on norm | In accordance with ISO 23696-1 2023-02 – Analogous to ISO 7890-1, DIN 38405 – D9 |
| Method | Nitrate - 2,6 -Dimethylphenol |
| NanOx N | No |
| NanOx Metal | No |
| Crack-Set | No |
| Sea water analysis | No |
| Remark | Measuring range on NANOCOLOR VIS II |
| Shelf life (from production) | 2 Year(s) |
| Storage temperature | 15–25 °C / 59–77 °F |
| Scope of delivery | Rugged box with 20 test tubes. Sufficient for 20 tests. |
| Gross weight (incl. packaging) | 464 g / 1.02 lbs |
| Packaging dimensions | 160 x 110 x 119 mm / 6.30 x 4.33 x 4.69 Inch |
| Hazardous material | Yes |
- Download Instruction / Anleitung / Manuel
- Download Pictogram / Piktogramm / Pictogramme
- Download Validation data / Validierungsdaten / Données de validation
- Download Betriebsanweisung (DE)
- Download Flyer NANOCOLOR analysis system (EN)
- Download Flyer Das NANOCOLOR Analysensystem (DE)
- Download Flyer Système d’analyse NANOCOLOR (FR)

Nitrate
Nitrate ions are present at varying concentrations (usually up to ≈ 20 mg/L) in ground and surface waters, as well as in municipal and industrial wastewaters. They are present almost exclusively in dissolved form in water samples. Nitrate levels of 15–50 mg/L indicate anthropogenic influences. They enter municipal wastewaters e.g. as the end product of nitrification.
How to perform the NANOCOLOR Nitrate tube test
General information
Nitrification is the bacterial oxidation of ammonia and other nitrogen-containing organic compounds. These emerge in large quantities as human and animal excretions or from decay processes of organic substances. Nitrates in surface and groundwaters can also originate from water-soluble components of artificial fertilizers.
Nitrate and the other nitrogen parameters ammonium and nitrite, are thus a measure for the contamination of a water body. It is important whether an increased nitrate content is linked to similarly increased ammonia and nitrite concentrations to evaluate the self-purification capacity of a water body.
If this is not the case, the self-purification capacity is sufficient for the mineralization of organic matter. The nitrate concentration is one of the most important chemical parameters to check the quality of drinking water. The EC guide value is 25 mg/L.
Detailed information about the nitrite and ammonium concentration as well as their relationships is essential for a proper assessment of drinking water. So-called reducing conditions are present if high ammonium and low nitrate levels are observed e.g. in a (ground)water polluted with nitrogen compounds. This nitrate reduction is effected among others by bacteria and fungi Streptomyces). Under oxygen-deficient conditions (O2 < 5 mg/L), nitrate (NO3–) is first reduced to nitrite (NO2– ), which is then degraded further e.g. to elemental nitrogen gas (N2). Other bacteria, by contrast, reduce nitrite (NO2– ) to ammonium (NH4+). The conditions are opposite in native, oxygen-rich groundwater. Here oxidation of ammonium (NH4+) and nitrite (NO2–) to nitrate (NO3–) by nitrogen bacteria (Nitrosomonas, Nitrococcus, Nitrobacter) takes place.
There is always pollution, if high nitrate concentrations cannot be attributed geologically to natural saltpeter deposits (especially in case of presence in groundwater).
Nitrate and nitrite are widely used as additives in the preparation of meat products (“curing”). On the one hand, the shelf life is extended by inhibition of putrefying bacteria. On the other hand, the heat- and storage-stable red curing color is formed (generation of the typical cured flavor), by the addition of nitric oxide (NO) to the muscle pigment myoglobin to form nitrogen oxide myoglobin.
The pollution of groundwater by nitrate is a serious deterioration of the environmental quality. Elevated nitrate levels have a negative impact on the ecology of the waters. They can also lead to a lower drinking water quality and thus to negative health effects. In the organism, nitrate can be converted, among other things, to nitrite, which inhibits Oxygen transport by the red blood pigment (hemoglobin).
Nitrate is primarily almost non-toxic (gastric inflammation usually occurs only at levels > 500 mg/L NO3–). The dangers from nitrates arise from the fact that they are partially converted to nitrites by bacteria in the body (Nitrite NO2– ). Tertiary conversion products of nitrate (in the human body, from amines and nitrite) can be N-nitroso compounds, which are classified as carcinogenic. Nitrate, depending on the dose, inhibits the iodide transport mechanisms of the human organism.
The notation of nitrate is NO3–, but often NO3-N is used instead. The difference is that in NO3-N only the mass of nitrogen is taken for account. The mass of oxygen in the nitrate is disregarded. The conversion factor is 4.43, which results from the large mass difference of nitrogen and nitrate
Nitrate is a pollution indicator and one of the most important chemical parameters for the control of drinking water quality.
With regard to human nutrition, especially some strongly nitrate-containing vegetables such as spinach, soy beans, chard, beets or radishes are to be named.
Reaction basis
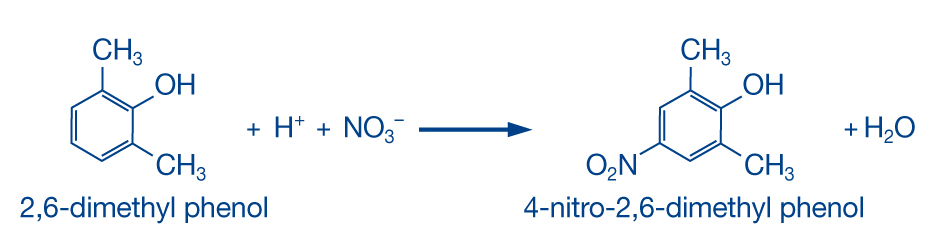
The determination reacts analogously to DIN 38405-D9-2 and ISO 7890-1, the photometric determination as 4-nitro-2,6-dimethyl-phenol.
The reaction uses 2,6-dimethylphenol in a mixture of sulfuric acid and phosphoric acid. Direct nitration of dimethylphenol results in the formation of 4-nitro-2,6-dimethylphenol, depending on the nitrate content of the sample.
Sample preservation
By adjusting the pH to 1-2 with sulfuric acid, the sample can be stored for up to 7 days (storage vessel: PE bottle). Ideally, storage and transport are carried out at 4 °C in the dark.
Tips & tricks
Common sources of error
Insufficient dissolution of the reagents causes the risk of findings below the actual value.
Background information
The filling level of the cuvette in standard tests must be at least half of the maximum
due to the beam path of the spectrophotometers.
The NANOCOLOR Nitrate tube tests meet the requirements of ISO 23696-1:2023-02: Water quality — Determination of nitrate in water using small-scale sealed tubes — Part 1: Dimethylphenol colour reaction.
This standard method specifies the determination of nitrate in natural water, drinking water and wastewater with measuring ranges from 0.10–225 mg/L NO3-N.
These standards provide clear guidelines and procedures for accurately measuring of nitrate in water samples. By adhering to these standards, our customers can be sure that our NANOCOLOR test kits are precise, reliable and comparable.
Sea water suitability
NANOCOLOR nitrate tests are only partially suitable for seawater analysis, which is due to chloride interferences. Chloride can be removed either by dilution or by use of partridges for chloride elimination (REF 963 911).
pH
The pH values of the sample solution stated in the package inserts must be complied with. If necessary, adjust the pH with sulfuric acid or sodium hydroxide.
Reaction temperature
The temperature of the sample should be in the range of 18–30 °C. Especially at lower
temperatures, the reaction proceeds much more slowly and may therefore lead to
lower apparent results.
Interferences
- Oxidizing substances may lead to lower apparent results or even completely inhibit the reaction depending on their concentration.
- Chlorine > 10 mg/L interferes.
- Nitrite interferes from 1 mg/L (identical underlying reaction). Nitrite must be determined in advance and removed before the measurement. Nitrite is eliminated by the addition of sulfamic acid (REF 918 973) [1 measuring spoon to 10 mL of sample solution, wait with the determination of nitrate for 10 min. The pH of the solution should be in the range of 2–3, otherwise adjust with sulfuric acid].
- Organic colloids, humic acids, colored heavy metal ions as well as oxidizing and reducing substances interfere.
- Nitrate analysis is generally disturbed by peroxides which can cause a rust-brown color.
- The standard test Nitrat Z has a very sensitive measuring range and is based on the reduction method. The test cannot be applied if any other reducible substances are present.
- Further interfering ions are listed in the instruction leaflets.
Turbidity
Turbid samples have to be filtered; turbidity leads to incorrect results: For coarsely dispersed turbidities, use qualitative filter paper (e.g. MN 615), for moderately dispersed turbidities, use glass-fiber paper (e.g. MN 85/70 BF) or membrane filtration set GF/PET 0.45 μm, for finely dispersed turbidities, use membrane filtration kit 0.45 μm or GF/PET 0.45 μm.